-
Overview
-
Assays
-
Case Study
-
Experience
-
FAQs
-
Related Resources
-
Related Services
Overview
Drug metabolism is the process of structural transformation of drugs under the catalysis of drug-metabolizing enzymes after absorption and distribution in the body. It is the major elimination route from the body for most drugs. The drug metabolic reactions can be classified into two types: phase I and phase II. Phase I metabolism, such as oxidation and reduction, involves adding functional groups to or exposing the functional groups from a molecule. Phase II metabolism involves conjugation reactions, such as glucuronidation and sulfonation. Appropriate biological matrices, such as hepatocyte, plasma, whole blood, microsome, S9, etc., can be selected according to the properties of drugs. By incubating the compound and biological matrices at different time points, the remaining at each time point is obtained, and the parameters such as half-life and intrinsic clearance are calculated.
Learn More

Assays
Case Study
-
-

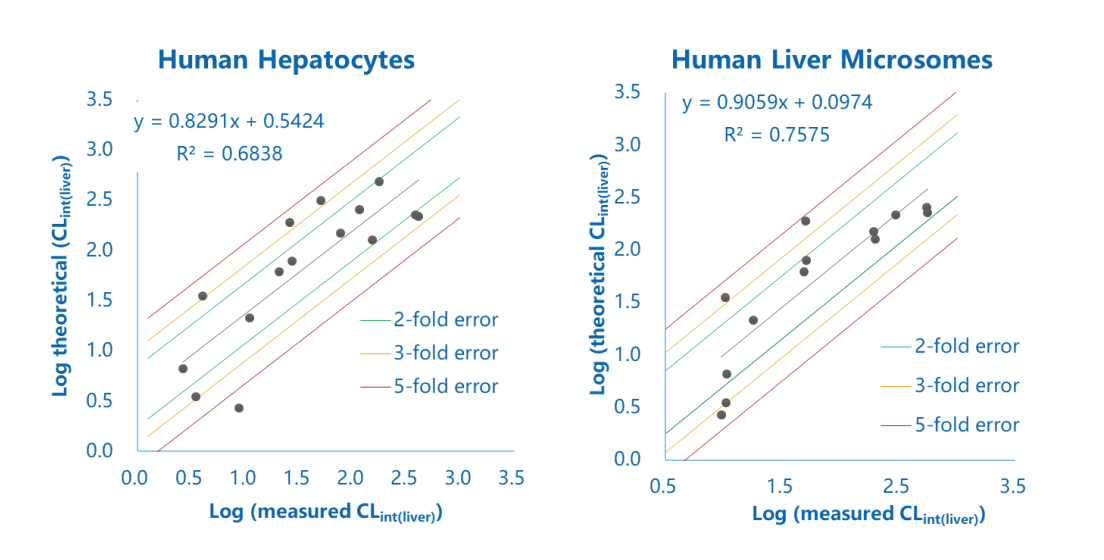
Logarithm plot of measured and theoretical CLint (liver) of commercial compounds obtained from human liver microsome and hepatocyte stability assays
The figure showed the in vitro and in vivo correlation of liver intrinsic clearance of 10+ drugs. The abscissa was the liver intrinsic clearance measured by an in vitro liver microsomal or hepatocyte stability assay. The ordinate was the liver intrinsic clearance calculated from the in vivo clearance reported in the literature. More than 60% of the compounds were within the 2-fold error range, and the correlation between in vitro and in vivo clearance was good.
-
Experience
-
18
Years
-
200K+
Compound* species screened per year
-
≤ 5
TAT ≤ 5 Days
FAQs
Related Resources




-


Methods and Strategies for In Vitro Metabolism Studies of Oligonucleotides
ArticlesJan 08, 2025Learn More -


In Vitro Metabolic Stability Evaluation of ADCs in Plasma and Whole Blood
PostersOct 17, 2024Learn More -


Establishment of Two In Vitro Glutathione Conjugation Models and Their Application in Covalent Drugs
ArticlesOct 12, 2024Learn More -


8 In Vitro Systems for Oligo Metabolism Study: Pros and Cons and Selection Suggestions
ArticlesAug 25, 2024Learn More -


Predicting In Vivo Metabolic Clearance Using In Vitro–In Vivo Extrapolation (IVIVE) Model: Why and How?
ArticlesAug 08, 2024Learn More -


Establishment of the Relay Method for Low Turnover Compounds in Hepatocyte Stability Assays
PostersApr 03, 2024Learn More -


Development of a Plasma Stability Assay of Oligonucleotides
PostersFeb 12, 2024Learn More -


Exploring Metabolic Stability: In Vitro Metabolic Models for Slowly Metabolized Compounds
BlogsJan 12, 2024Learn More -


Metabolic Stability Studies: How to Study Slowly Metabolized Compounds Using In Vitro Models
ArticlesDec 28, 2023Learn More -


How to Address the Challenges of PROTAC Metabolism
BlogsJul 13, 2023Learn More -


Research on PROTAC Metabolism: Strategies and Main Approaches
ArticlesJun 25, 2023Learn More -


In Vitro/Vivo Characterization of ADC Drugs by Immunocapture and High-resolution Mass Spectrometry
PostersMay 19, 2023Learn More
Stay Connected
Keep up with the latest news and insights.